Review Ja P O N E S Q U E Foundation Brush
Introduction
Soil organic carbon (SOC) is a major carbon reservoir in the terrestrial ecosystems and its slight change would have a considerable touch on on the global carbon (C) balance (Cotrufo et al., 2015). Litter and root exudation provide a large amount of labile substrate to soil microorganisms (Paul, 2016), mediating C cycling in the terrestrial ecosystems (Cheng et al., 2014; Paul, 2016). Kickoff, inputs of labile C can greatly enhance native SOC mineralization, which is termed as priming outcome (PE) (Blagodatskaya & Kuzyakov, 2008; Kuzyakov, 2010; Blagodatskaya et al., 2007). Second, role of the added labile C can be retained in the soil to compensate the SOC loss caused by PE (Ohm, Hamer & Marschner, 2007; Liang & Balser, 2012; Cotrufo et al., 2013; Cotrufo et al., 2015; Liang, Schimel & Jastrow, 2017). Hence, labile C input studies should consider net C balance between the retained C and the primed C (Griepentrog et al., 2014). Additionally, the primed C was found to be originated from stable SOC (Fontaine et al., 2007; Blagodatskaya et al., 2011; Derrien et al., 2014). If the retained C is less stable than the primed C, the long-term consequence of C input on SOC storage remains uncertain. Therefore, it is necessary to investigate how much exogenous C tin be sequestrated in soil and the stabilization of the new sequestrated C (Powlson et al., 2014; Janzen, 2015). This new cognition can show new insights into our authentic assessment of the impact of labile C input on soil C puddle.
Current studies suggested that the new sequestrated C has faster turnover time and lower stability (Derrien et al., 2014; Van Groenigen et al., 2017). However, the reason underlying the phenomenon was poorly understood. It is necessary to reveal the SOC stabilization mechanism for the authentic evaluation of SOC stability. Soil fractionation analysis is frequently used to written report the stabilization mechanisms of SOC past separating SOC into different C fractions. These SOC fractions correspond to different mean residence times and stabilization mechanisms (Christensen, 1992; Christensen, 2001; Vi et al., 2002; Von Lützow et al., 2007). Therefore, studying the dynamics of labile C incorporation into these soil fractions tin elucidate the new C stabilization mechanisms and evaluate its stability.
Deep soil (below 30 cm) contains more half of the total soil C stocks (Rumpel & Kögel-Knabner, 2011). The response of deep soil to labile C input is thus important to terrestrial C balance. Previous studies generally found that deep soil had a stronger PE than surface soil due to its soil physio-chemical and microbial properties (Tian et al., 2016; Wang et al., 2014b). Information technology is urgent to know how much and how long the exogenous C can be retained in soil to evaluate the long-term C sequestration in forest ecosystems.
Soil C cycling nether labile C input can be mediated by nitrogen (N) availability (Chen et al., 2014; Qiu et al., 2016). Many studies mostly found that higher N availability could reduce soil PE by regulating soil microbial activity and metabolic efficiency (Wang et al., 2014a; Wang et al., 2014b; Chen et al., 2019). The changed microbial properties might farther affect exogenous C mineralization and C sequestration. Field researches showed Due north add-on could significantly regulate SOC residual mainly through indirect effects on vegetation C input (Liu et al., 2019). However, the straight bear on of N addition on new C sequestration was unclear.
In society to identify the effects of soil depth and N availability on the soil C sequestration potential and the stability of the new sequestrated C, surface soil (0–10 cm) and deep soil (30–threescore cm) from a subtropical forest were incubated for xx days with the addition of xiiiC labeled glucose and NHfourNOiii. Soil COii efflux rates and δ13C values were measured during the incubation. SOC was fractioned into vii fractions and collectively divided into three functional pools past a combination of density, particle and chemical methods to elucidate C stability. Previous studies showed that SOC in deep soil was farther abroad from C saturation than that in surface soil, and deep SOC was less decomposable than surface SOC (Stewart et al., 2008; Poirier et al., 2013; Derrien et al., 2014; Van Groenigen et al., 2017). Therefore, we hypothesized that the deep soil could retain college proportion of exogenous C than surface soil, and the new C in the deep soil was more than stable. We also hypothesized that N addition could increment the new C sequestration and stability.
Material and methods
Soil collection
Soil samples used in this experiment were collected in an evergreen and deciduous broad-leaved mixed wood located at Badagongshan National Research Reserve (29°46.04′North, 110°5.24′E) in Sangzhi county, Hunan Province. More detailed site clarification could be seen in Tian et al. (2016). Field sampling was verbally permitted past Zhirong Gu, who is a worker at the Badagongshan National Research Reserve.
The soils were collected from two depth intervals: 0–10 and 30–60 cm (representing the surface soil and deep soil, respectively) through earthworks a trench. The 10–30 cm soil included mixed samples from mineral A layer, transition layer, and Bts layer. Therefore, nosotros did not consider this layer in our study. The soils were homogenized and and so sieved (two mm). The coarse roots and visible residues were picked out during the sieving. Soils were stored beneath 4 °C until further incubation. SOC and TN contents were 131.7 and 7.9 mg g−1 in surface soil, and was 35.seven and two.7 mg g−1 in deep soil (Tabular array 1). The clay contents were 22.6% and 39.six% in surface soil and deep soil, respectively.
Table 1:
Characteristics of the soil samples.
| Variables | Surface soil (0–10 cm) | Deep soil (30–60 cm) |
|---|---|---|
| Then (mg thou−i) | 131.seven | 35.vii |
| Total N (mg k−1) | 7.9 | 2.7 |
| C/N | 16.7 | xiii.4 |
| δ 13C (‰) | −28.2 | −25.8 |
| LF (%) | four.04 | 0.56 |
| Sand (%) | 3.18 | iii.98 |
| Silt (%) | 70.17 | 55.86 |
| Clay (%) | 22.61 | 39.threescore |
| WEOC(%) | 0.33 | 0.33 |
| LF C (%) | x.14 | 4.eleven |
| Sand C (%) | 0.72 | 2.38 |
| H-silt C (%) | 28.17 | 33.78 |
| NH-silt C (%) | 36.58 | 17.11 |
| H-clay C (%) | 12.03 | 29.11 |
| NH-clay C (%) | 12.03 | 13.18 |
| MBC (µg C g−1 soil) | 2061.8 | 154.7 |
Experimental design and soil incubation
The incubation experiment included three treatments: soil without addition (Control), soil with glucose improver (Glu), and soil with combined additions of glucose and N (Glu+N) with six replicates. For each soil, the corporeality of added glucose-C corresponded to 100% of soil microbial biomass C (MBC) (surface soil, 2061.8 µg C g−1 soil; deep soil, 154.seven µg C g−one soil). This quantity has been widely adopted in priming experiments (Li et al., 2017; Chen et al., 2019). The amount of Due north improver corresponded to the C: N = 10 of the added substrate with NH4NO3 (surface soil, 206.18 µg N g−1; deep soil, 15.47 µg N thousand−1 soil). The command received the same corporeality of distilled water. For incubation, about 60 grand soil (equivalent to 30 g dry out soil) for each replicate was placed into an individual 250 mL Erlenmeyer flask. Soil samples were pre-incubated at 20 °C for 5 days in the dark condition. Later pre-incubation, each replicate of Glu and Glu+N treatments was amended with aliquots of glucose solution (uniformly labeled, δ13C = two,000‰) with or without North source (NH4NO3). For the command handling, equivalent distilled H2O (3 mL) was added to the soil samples. Then, soil samples were incubated in dark nether the ambient air condition for 20 days. During the incubation, the soil water content was maintained at 65% water-property chapters by weighing the flask every 4 days. Soil CO2 efflux rates were measured on days 0, 1, 2, 3, iv, vii, 10, 15 and 20. Afterwards the xx days incubation, the COtwo efflux charge per unit tended to be abiding. Thereafter, destructive samplings were conducted. Three replicates were used to mensurate the MBC, and the other iii replicates were oven stale at 60 °C to clarify SOC fractions.
Measurements of CO2 efflux rates
At each measurement time, three of the replicates were randomly chosen to measure CO2 efflux rates past an infrared gas analyzer (IRGA; EGM-4, PP Systems, USA). Another three replicates of each handling were chosen to determine δxiiiC of the released CO2 by Carbon isotope analyzer (912-0003, LGR, USA). The detailed procedures could be seen in Tian et al. (2016).
Measurement of MBC
MBC was adamant past the chloroform fumigation extraction method (Vance, Brookes & Jenkinson, 1987). Soil samples were divided into ii 10 k fresh soil. I was fumigated with ethanol-complimentary chloroform for 24 h followed by extraction with 0.05 mol 50−1 Thousand2SO4 (shaken for 30 min) and the other was extracted immediately with 0.05 mol L−1 K2SO4 (shaken for xxx min). The extraction was and so adamant by Total Organic Carbon Analyzer (Vario TOC, Elemental, Deutschland). The MBC (difference in KiiSO4-extractable C betwixt fumigated and not-fumigated samples) were corrected using universal conversion factors of 0.45 (Garcia-Pausas & Paterson, 2011). The extractable C content for the non-fumigated soil samples was considered as dissolved organic carbon (DOC).
SOC fractionation
Soils were fractionated using a combination of density, particle and chemical protocol, adapted and modified from Denef et al. (2013) and Half dozen et al. (2002) (Fig. S1). Before SOC fractionation, h2o-extractable organic carbon (WEOC) was extracted by shaking 5 g of dried soil (<2 mm) in 20 mL of deionized h2o on a shaker for 2 h. After extraction, the solid residue was separated into light fraction, sand, silt and clay. Briefly, the oven-dried (60 °C) solid residue soil was placed in a fifty mL centrifuge tube and 25 mL of NaI solution with a density of 1.85 yard cm−three were added. Tubes with the soil-NaI mixture were shaken in a shaker table at 300 rpm for ii h. So, the samples were centrifuged and the floating light fraction (LF) (LF < i.85 grand cm−three) was transferred onto the microfiltration membrane and filtered under vacuum. The remaining heavy fraction was washed with deionized water to remove NaI and so sieved through a 53 µm screen to separate the sand (>53 µm) fraction from the silt and clay. Silt and clay fractions were separated through wet centrifugation (127 g for 7 min for silt, and 1,730 thousand for 15 min for dirt, and then farther hydrolyzed in 6 mol L−1 HCl at 95 °C for 16 h. The C in the suspension and residue were considered as hydrolyzable C fraction (H-silt, H-clay) and not-hydrolyzable C fraction (NH-silt, NH-clay), respectively.
All solid fractions were oven-stale at 60 °C prior to weighing. C content and its δ13C in the solid fractions were measured on a thermal combustion elemental analyzer (Fisher Flash 2000, Thermo Fisher, USA) interfaced with a stable isotope Mass Spectrometer (Delta 5 Reward, Thermo Finigan, Frg). C content in the water solution was determined by TOC (Vario TOC, Elemental, Frg).
The δ13C of WEOC and Physician was adamant by sodium persulfate oxidation to transform the liquid into gas modified by Midwood et al. (2006) and Garcia-Pausas & Paterson (2011). Briefly, 10 mL water solution was added into a 250 mL reaction canteen and then 100 µL of 1.3 mol L−one phosphoric acid solution was added to remove inorganic C from the solution. Later on that, 200 µL of 1.05 mol L−ane sodium persulfate was added. In the bare control, x mL ultrapure water was added, and the other steps were the same. The reaction canteen was and then capped and flushed with CO2-complimentary air. The sample was then heated in a 90 °C water bath for 30 min to promote the oxidation reaction. The gas in the canteen was transferred into airbag to determine the COii isotope value by Carbon isotope analyzer (912-0003, LGR, United states).
The seven fractions were further divided into three functional C pools based on the supposed relationship between the soil fractions and the stabilization mechanisms: not-protected C pool, chemically protected C pool and biochemically protected C pool modified from 6 et al. (2002). The constitute of three functional C pools was shown in Fig. S1.
Calculations
The soil CO2 efflux rate derived from native SOC and glucose-C during incubation was calculated in Tian et al. (2016).
In the incubation experiment, the amount of primed C and relative magnitude of PE was calculated equally follows: (one) (2)
where AR primed is the amount of primed C during the incubation. AR SOC(treatment) is the soil cumulative released-C derived from SOC in the treatment with glucose addition, AR SOC(command) is cumulative released-C in control.
The percentage (f) of glucose-derived C in majority and soil fractions was calculated according to simple isotopic mixing model: (3)
where the δ13 C handling and δ13 C command were the δthirteen C value of each C fractions in the treatments with glucose addition and control, respectively. δ13 C Glu is the isotopic signature of the glucose C added to the soil samples. The amounts of new C in majority and soil fractions were calculated according to C content in majority soil and soil fractions likewise as f.
The sequestration potential for glucose-C (proportion of glucose-C retained in soil) was calculated as follows: (4)
where C retained is the corporeality of glucose-C retained (new C) in soil, C add-on is the amount of added glucose.
The content of net sequestrated C was calculated as the divergence between C retained and AR primed . The net C sequestration potential was calculated as the content of internet sequestrated C per unit of added glucose-C: (5)
The microbial metabolic caliber (qCO 2) was calculated as the COtwo efflux rate per unit of MBC on day 20.
Statistical analysis
The differences in CO2 efflux rate, isotope signature of each soil fraction, and qCO2 among the three treatments and two soil depths were analyzed using two-mode ANOVA. The effects of soil depth and N availability on the amount of primed C, the relative magnitude of PE, the amount of retained glucose C, the sequestration potential, the net C sequestration, and the distribution of new C in each soil fraction were compared using two-way ANOVA. Tukey's postal service hoc test was used to identify pregnant differences at p <0.05. The distributions of new C and native C in each soil fraction were compared through independent t test. Master component analysis (PCA) was used to clarify the distribution of new C and native C in soil fractions. Permutational multivariate analysis of variance (PERMANOVA) with Euclidean distance matrixes was used to evaluate the difference of distribution between new C and native C under the ii soil depths. Statistical analyses were performed using SPSS version 21.0. The PCA was conducted using the "vegan", "lattice", "permute" packages in R version 3.5.1. The PERMANOVA analysis was performed using By 3.
Result
SOC mineralization and glucose-C retentivity
Glucose addition increased the CO2 efflux charge per unit (Fig. S2). I part of the increased CO2 efflux was from the added glucose (glucose-derived CO2), while the other part was from the native SOC (native SOC-derived COtwo). The amount of primed C and the relative magnitude of PE were significantly affected past soil depth and N availability, and no interaction effect between soil depth and N availability was observed (Tabular array ii and Fig. S1). The relative magnitude of PE was higher in deep soil than that in surface soil, and North addition significantly decreased them in both soil depths.
Later 20 days of incubation, the amount of retained glucose-C and the C sequestration potential were significantly affected by soil depth, simply showed no difference between Glu and Glu+N treatments in both soil depths (Tabular array 2). The retained glucose-C was averaged 875.3 µg g−1 and 78.three µg g−1 in surface soil and deep soil, respectively (Tabular array two). Deep soil had significantly college C sequestration potential (49.nine ± 1.1%) than surface soil (43.half-dozen ± 0.1%).
The net C sequestration was quantified by calculating the trade-off between the retained glucose-C and the primed C. At the end of twenty days incubation, the retained glucose-C was higher than the amounts of primed C, resulting in overall positive cyberspace C sequestration for both soil depths (Tabular array ii). The corporeality of net sequestrated C and net C sequestration potential was significantly afflicted by soil depth and N availability, and significant interaction effects were besides observed. The amount of net sequestrated C was significantly higher in surface soil than that in deep soil, but deep soil had significantly higher net C sequestration potential than surface soil. N add-on could increase the amount of net sequestrated C and cyberspace C sequestration potential for both soil depths (Table 2).
Table 2:
The amount of primed C, the relative PE, the amount of retained glucose C, the C sequestration potential, the amount of cyberspace retained C and the net C sequestration potential after 20 days incubation.
| Parameters | Surface soil | Deep soil | |||
|---|---|---|---|---|---|
| Glu | Glu+N | Glu | Glu+N | ||
| Added glucose-C (µg C g−1 soil) | 2061.eight | 2061.8 | 154.7 | 154.7 | |
| Primed C (µg 1000−1) | 257.1 ± 14.2Aa | 57.7 ± 9.5Ab | 17.0 ± 0.3Ba | 8.9 ± 0.2Bb | |
| Relative PE (%) | 28.ii ± 0.01Aa | 6.3 ± 0.01Ab | 36.vi ± 0.0Ba | 19.1 ± 0.0Bb | |
| Retained glucose C (µg g−1) | 872.half dozen ± 2.5Aa | 878.0 ± 2.5Aa | 77.ii ± 1.7Ba | 79.5 ± three.9Ba | |
| The C sequestration potential (%) | 43.vi ± 0.1Aa | 43.9 ± 0.1Aa | 49.ix ± ane.1Ba | 51.4 ± 4.3Aa | |
| The amount of internet retained C (µg g−one) | 615.5 ± 14.2Aa | 820.iii ± 9.5Ab | 60.1 ± 0.3Ba | 70.6 ± 0.2Bb | |
| Net C sequestration potential (%) | 29.9 ± 0.7Aa | 39.8 ± 0.5Ab | 38.ix ± 0.2Ba | 45.6 ± 0.1Bb | |
C distribution in soil fractions
Glucose addition significantly increased the isotope signature of majority soil and the seven fractions (Fig. 1). Compared to Glu treatment, Glu+N increased the isotope signature of LF, sand, WEOC, and NH-clay fractions in both soil depths, but had no significant event on bulk soil.
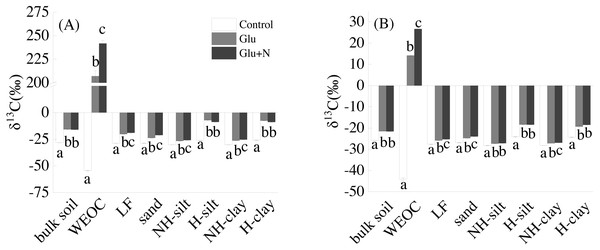
Effigy i: The isotope signatures of bulk SOC and SOC fractions in surface soil (A) and deep soil (B).
Results are ways ± SE (n = iii). Dissimilar messages indicated a significant deviation among the three treatments in the same fraction.The distributions of the new sequestrated C in soil fractions were significantly afflicted by soil depth and N availability, and interaction effects betwixt soil depth and N availability were not observed (Table 3 and Table S2, Figs. two and 3). Compared to the deep soil, the new C in surface soil was more distributed in WEOC (8.5% VS five.1%), LF fraction (6.9% VS 1.9%) and silt fraction (58.ix% VS 43.viii%). In contrast, the proportion of new C associated with clay fraction was higher in deep soil than that in surface soil. When silt and clay fractions were further hydrolyzed by acid, the new C associated with silt and clay fractions in deep soil was more acid hydrolyzable (91%) than that in surface soil (80%). North addition slightly increased the proportion of new C incorporated into WEOC, sand fraction and NH-clay fraction for both soil depths, whereas showed no significant effects on other fractions. According to the PERMANOVA analysis, the new C distribution blueprint was significantly affected by soil depth (p = 0.0009), but not N addition (p = 0.14).
Table 3:
The outcome of soil depth and Due north addition on the new C distribution in soil fractions.
| Treatment effect | Soil fractions | Silt and clay fractions | |||||||
|---|---|---|---|---|---|---|---|---|---|
| WEOC | LF | sand fraction | silt fraction | clay fraction | H-silt | NH-silt | H-clay | NH-clay | |
| Depth | *** | *** | * | *** | *** | ns | *** | *** | *** |
| Nitrogen | * | ns | * | ns | ns | ns | * | ns | *** |
| Depth*Nitrogen | ns | ns | ns | ns | ns | ns | ns | ns | ns |
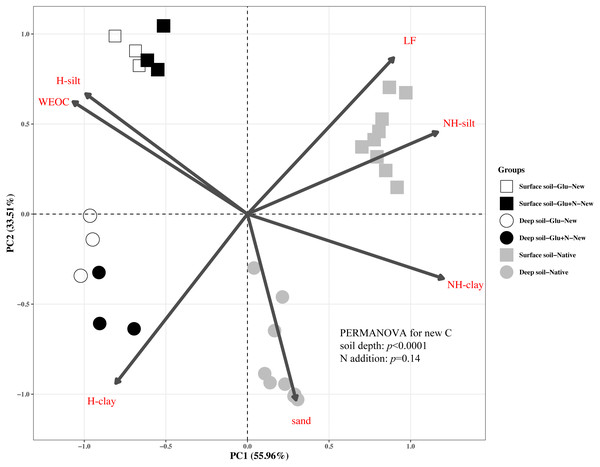
Figure ii: Master Component Assay (PCA) of the distribution patterns of new and native C in surface soil and deep soil.
Square represents the surface soil and circle represents the deep soil. Hollow and blackness solid denotes new C under Glu and Glu+N treatments, respectively, and grey solid denotes native C. The results of PERMANOVA show the effects of soil depth and North addition on the new C distribution pattern.The 7 SOC fractions were grouped into three functional SOC pools for further analysis (Table iv). Compared to deep soil, the new C in surface soil was more distributed in non-protected C puddle (fifteen.8% VS viii.4%) and biochemically protected C puddle (15.5% VS 7.0%) and less distributed in chemically protected C pool (58.viii% VS 74.seven%). N addition significantly increased the proportion of new C incorporated into non-protected C pool and biochemically protected C pool in deep soil (p < 0.05), and increased proportion of the new C incorporated into biochemically protected C puddle (p < 0.05) in surface soil.
Table four:
The distribution of new C and native C in the three functional SOC pools.
| SOC pools | Treatments | New C% in bulk new C | Native C% in bulk C | |||
|---|---|---|---|---|---|---|
| Surface soil | Deep soil | Surface soil | Deep soil | |||
| Non-protected C pool | ||||||
| Glu | 14.4 ± 0.3Aaa | seven.6 ± 0.4Ba | 11.viii ± 0.3 | 6.4 ± 0.v | ||
| Glu+N | 17.1 ± i.1Aaa | 9.1 ± 0.3Bb | ||||
| Chemically protected C puddle | ||||||
| Glu | 60.ii ± 0.4Aaa | 75.eight ± 2.6Baa | 45.ane ± 0.9 | 58.1 ± 1.1 | ||
| Glu+N | 57.three ± 0.9Aba | 73.six ± 3.0Baa | ||||
| Biochemically protected C pool | ||||||
| Glu | 14.one ± 0.1Aaa | 6.ane ± 0.5Baa | 51.9 ± 0.7 | 27.3 ± 0.seven | ||
| Glu+Due north | xvi.9 ± one.0Aba | vii.eight ± 0.3Bba | ||||
The distribution of the new C in the seven fractions likewise differed significantly with native C for both soil depths (Fig. ii). Compared to the native C, the new C was more distributed in not-protected C puddle and chemically protected C pool, and less distributed in biochemically protected C pool (p < 0.05).
Soil qCO2
The qCOtwo in deep soil was significantly lower than that in surface soil (p < 0.05) (Fig. 4). In deep soil, treatments of Glu+Northward and Glu had significantly lower qCO2 than control treatment. In surface soil, qCOtwo showed no significant departure amid the three treatments.
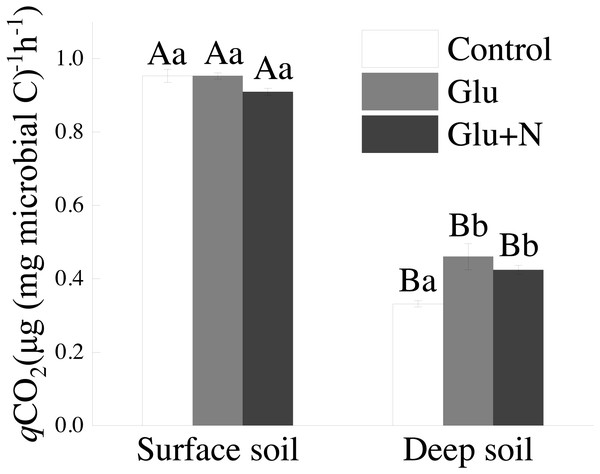
Effigy 4: The effects of glucose and N addition on q CO2.
The lowercase letter of the alphabet indicated the divergence among the different treatments in the same soil depth and upper-case letter messages indicated a meaning difference between surface soil and deep soil in the same treatment. These values are means ± SE (northward = 3).Discussion
The retention of glucose-C
Labile C input not only altered the native SOC mineralization, but also resulted in new C formation and sequestration (Cotrufo et al., 2015; Haddix, Paul & Cotrufo, 2016). Previous studies have demonstrated that added glucose can exist completely mineralized and assimilated past microbes within five–7 days (Coody, Sommers & Nelson, 1986; Baldock et al., 1989; Lundberg, Ekblad & Nilsso, 2001; Zhang et al., 2015). First, microbes utilized the glucose for biomass product through the vivo turnover pathway. Second, the glucose-derived microbial residues and their metabolites could be selectively absorbed by soil minerals, and so incorporated into organo-mineral complexes (Liang, Schimel & Jastrow, 2017). Furthermore, the contents of glucose-derived Physician (extracted by 0.05 mol Fifty−i M2SOfour for the fresh soil samples at the end of the 20 days incubation) accounted for 0.07%–0.thirteen% and 0.3%–0.56% of the added glucose in surface soil and deep soil, respectively (Fig. S3), which indicated that little free glucose left in the soil. Thus, nosotros considered that the retained glucose-C mostly existed equally live MBC, microbial necromass or microbial metabolites (Liang, Schimel & Jastrow, 2017; Wang et al., 2020), and soil microorganisms contributed a great part on the C sequestration, especially for the simple C sources (Wardle, 1992; Xu, Thornton & Post, 2013; Liang, Schimel & Jastrow, 2017). Since 80% of the retained glucose-C could incorporate into soil particles eventually (Griepentrog et al., 2014), the retained glucose-C in the soil was considered equally new sequestrated C at the end of incubation.
Our results suggested that deep soil could retain more proportion of exogenous C than surface soil, which supports our commencement hypothesis. This could be explained by microbial C utilize efficiency. qCOtwo has been used every bit a proxy of microbial C use efficiency. The lower qCOii values in deep soil (Fig. 3) corresponded to higher C resource allotment to MBC than to respiration losses, indicating a higher C sequestration potential for glucose-C (Chen et al., 2018). Previous studies also showed that the qCO2 decreased with soil depth in forest soils (Spohn & Chodak, 2015). However, Spohn et al. (2016) suggested that CUE varied little with soil depth in two forests. The discrepancy may exist explained by the C and Due north availability or the differences in MBC and community composition (Spohn & Chodak, 2015). Additionally, SOC in deep soil was further abroad from C saturation than that in surface soil. According to the conceptual model of C saturation, the C-poor deep soil would probably take greater potential and efficiency to retain exogenous C (Stewart et al., 2008; Poirier et al., 2013).
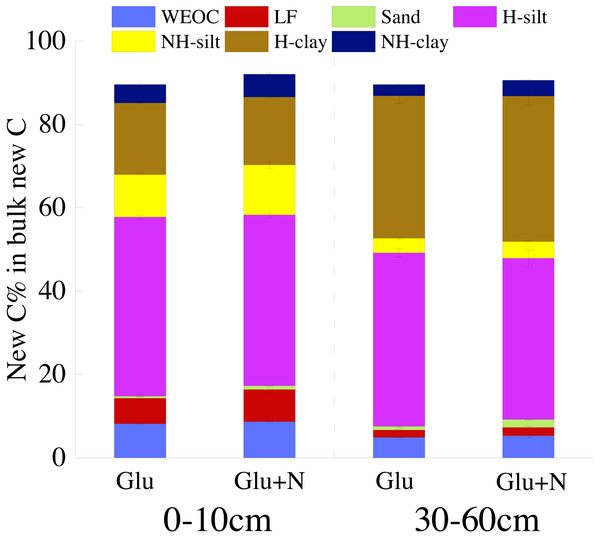
Figure 3: The distribution of new C in the measured soil fractions afterwards twenty days incubation.
N addition showed no effect on the amount of retained glucose-C, which was contrary to our 2d hypothesis. Information technology was probable that Due north addition did not significantly affect the microbial C employ efficiency, and then did not modify the proportion of new C sequestration. This issue concurred with the study that North addition did not affect the amount of litter-derived SOC in soil, and suggested that the high Due north availability in agroecosystem might not affect the C sequestration (Gentile, Vanlauwe & Vi, 2011).
The stabilization of new sequestrated C
To ameliorate evaluate the stabilization of new sequestrated C, we divided the SOC into different fractions. Labile C addition significantly increased the isotope signature of the soil fractions in both soil depths (Fig. 1), which indicated that glucose-C could be immobilized into all soil fractions. Nearly lxxx% of the new C was associated with silt and clay minerals for both soil depths. This outcome was in line with other researches, which constitute exogenous labile C could exist utilized by microorganisms and transformed into mineral-stabilized C as microbial necromass quickly (Bird, Kleber & Torn, 2008; Liang, Schimel & Jastrow, 2017; Garten & Wullschleger, 2000). Cotrufo et al. (2015) also suggested that 68% of the litter-derived C in soil was recovered in the mineral-associated silt and clay fraction in the early stage of litter decomposition. Additionally, there was 5–viii% of the new sequestrated C distributed in WEOC. The new C in WEOC was idea to be the labile C puddle and could be mineralized in the foreseeable hereafter. Overall, associated with soil silt and clay minerals were the main stabilization process for the new C.
Soil depths had a significant result on the distribution of new C in soil fractions (Fig. 2, Table 3 and Table S2). Compared to surface soil, a higher proportion of new C was stabilized by dirt minerals and a lower proportion of new C were stabilized by silt minerals in the deep soil. This could exist explained by a higher proportion of clay particles and a lower proportion of silt particles in the deep soil. New C in surface soil was more distributed in NH-silt (eleven.ane% VS three.7%) and NH-clay (4.nine% VS 3.3%) than that in deep soil, which may be attributed to the different microbial products and mineralogy composition in dissimilar soil depths (Kögel-Knabner, 2002; Silveira et al., 2008; Kallenbach, Frey & Grandy, 2016). Thus, the distribution of new C might partly depend on the soil properties.
Although N add-on had no effect on the total amount of glucose-C retained in soil, Due north addition could slightly increase the incorporation of glucose-C into WEOC and sand fraction. The higher distribution of glucose-C in WEOC and coarse fractions under North addition were besides reported by Griepentrog et al. (2014) and Hagedorn, Spinnler & Siegwolight (2003). The decreased turnover of these non-protected fractions under N addition may be responsible for the results (Gentile, Vanlauwe & Half-dozen, 2011). Northward addition could increase the N-rich microbial products which could be preferentially associated with the organo-mineral complexes rather than straight attached to the mineral surface (Kopittke et al., 2018). This could explain the higher non-hydrolyzable fraction in clay fractions nether N addition in both soil depths.
In this report, nosotros divided the SOC into three functional C pools, namely not-protected C pool, chemically protected C pool and biochemically protected C pool. The non-protected C puddle was not stable and could exist hands mineralized (Von Lützow et al., 2007; Battin et al., 2009; Kindler et al., 2011). Chemically protected C (H-silt and H-clay) is protected by association with mineral particles, where biochemically protected C is a non-hydrolyzable fraction that is stabilized by its inherent complex biochemical resistance through condensation and complexation reactions (Six et al., 2002). About 70% of the new C distributed in chemically protected puddle revealed that chemical protection contributes nigh to C stabilization. Compared to surface soil, more than new C distributed in clay fraction and less new C distributed in non-protected C pool for deep soil suggested that new C in deep soil was more stable than that in surface soil, which supports our first hypothesis. This consequence was consequent with previous studies that SOC in deep soil was more stable due to its microbial origin and intimate association with minerals (Rumpel & Kögel-Knabner, 2011). Although N addition increased the distribution of new C in non-protected C pool and biochemically protected C puddle, PERMANOVA analysis indicated N add-on had little effect on the overall new C distribution pattern (Fig. 2). This upshot suggested that the result of N add-on on the new C stability was express in the short term.
Compared to the native C for both soil depths, the new C was distributed more in non-protected C puddle and less in biochemically protected C pool, indicating that the new C may exist less stable than native C. This consequence was consequent with previous studies that the new incorporated C were more decomposable than native SOC (Derrien et al., 2014; Van Groenigen et al., 2017). Solid-state nuclear magnetic resonance (NMR) spectroscopy analysis as well showed that the glucose-C was transformed more often than not into O-alkyl C with trivial into effluvious C (Baldock et al., 1989). Low stability of new C in soil may overestimate the potential of exogenous C sequestration in the short-term. Information technology'southward notable that nosotros here added the elementary decomposable glucose into soil, the proportion of exogenous C retained and its incorporation into SOC fractions might be different from the complex substrates (e.g., litter and root).
The residuum betwixt primed C and new C
We observed positive PE and new C sequestration in both soil depths. The furnishings of labile C input on soil C pool should exist evaluated in the context of net C sequestration (Qiao et al., 2014). In the present written report, both soil depths showed the positive net C sequestration indicated by the higher amount of new sequestrated C than the primed C in bulk soil. The cyberspace increase in SOC was about 35% of the added labile C which was very similar to the meta-analysis results in Liang et al. (2018). Although deep soil had a relatively college PE, the cyberspace C sequestration was higher than that in surface soil. In contrast with our results, previous studies showed that SOC puddle in deep soil did not increase nether the increased exogenous C input (Mobley et al., 2015). The reasons for these divergent results might be that the quantity and quality of the exogenous C and soil blazon were different. Recent studies had demonstrated that when exogenous C was low, the SOC replenishment from exogenous C could non compensate for the loss of native SOC (Xu et al., 2019). Notably, the amount of added glucose-C in this report was different between the 2 soils (corresponding 100% of soil MBC). When the same amount of glucose was added, the pattern of C sequestration potential between surface soil and deep soil would change and depend on the amounts of added substrates. Thus, the wide application of these results should be cautious. Future studies with more soil types are needed to investigate the outcome of quantity and quality of the exogenous C on net C balance.
Northward add-on could heighten positive net C sequestration through decreasing SOC mineralization (Table 2). The declined PE under Northward addition could be explained past "microbial nitrogen mining" hypothesis which assumes that an increase in N availability will reduce microbial activity in mining SOC (N-containing substrates in soil) to meet Due north requirement (Fang et al., 2018; Chang et al., 2019). The significant interaction effect of North and soil depth suggested that the surface soil tended to retain more cyberspace C under N addition. This result demonstrated that hereafter N degradation may favor soil C sequestration by reducing recalcitrant SOC degradation.
Our report suggested that deep soil had higher C sequestration potential than surface soil and N improver could amend the internet C sequestration, simply widely extrapolating of these results should be cautious. Beginning, all soil samples were sieved through a ii mm sieve, which might liberate some physical protected SOC and increase their accessibility to the microbes. The inverse soil environments might obscure the results. 2d, the xx-day incubation could not stand for the long-term soil C cycling process. Long-term experiments were needed to monitor the processes of C sequestration. Third, new C sequestration also varied with the quality and quantity of the exogenous C. Iv, compared to N availability, P availability was suggested to exist more important to soil C cycling in tropical and subtropical forests (Hui et al., 2019). The ignoring of P availability and CNP stoichiometry in this study would constrain the awarding of our results. Extending studies considering these multiple factors could better our understanding on C sequestration potential for subtropical forest soils.
Conclusion
In summary, labile C addition could result in positive PE in both soil depths, leading to a loss of native SOC. Additionally, labile C input could be sequestrated in soil and overcompensated the C loss induced by PE. Deep soil could sequestrate more than proportion of added glucose-C than surface soil, resulting in greater net C sequestration. N addition further increased the positive net C sequestration by decreasing native C mineralization rather than through increasing glucose-C retentivity. The C distribution in soil fractions suggested that the new C in deep soil was more stable than that in surface soil, and the new C was less stable than the native SOC. Deep soil could retain more proportion of exogenous C with higher stability, suggesting that the deep soil could play a greater part on the C sequestration and stabilization. The lower stability of the new C suggested the soil sequestration potential for exogenous C could be overestimated in short term studies. Futurity studies with long-term lab incubations and field studies are needed to explore the controlling factors that mediate net C residue and new C stability.
Boosted Information and Declarations
Competing Interests
The authors declare at that place are no competing interests.
Author contributions
Chang Liao conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Dong Li performed the experiments, prepared figures and/or tables, and canonical the final draft.
Lin Huang performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Pengyun Yue analyzed the data, prepared figures and/or tables, and approved the final draft.
Feng Liu conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Qiuxiang Tian conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the concluding typhoon.
Field Written report Permissions
The following information was supplied relating to field written report approvals (i.east., blessing body and any reference numbers):
Field sampling was verbally permitted by Zhirong Gu, who is a worker at the Badagongshan National Research Reserve.
Information Availability
The following data was supplied regarding data availability:
The raw measurements are bachelor in a Supplemental File.
Funding
This enquiry was supported by the Natural Scientific discipline Foundation of Red china (31870465, 31600377, 31700462). The funders had no role in study blueprint, information drove and analysis, decision to publish, or preparation of the manuscript.
References
-
The boundless carbon bicycle. Nature Geoscience 2 :598-600
-
Nitrogen availability regulates topsoil carbon dynamics afterwards permafrost thaw by altering microbial metabolic efficiency. Nature Communications 9 :3951
-
Does the addition of labile substrate destabilise old soil organic matter? Soil Biology and Biochemistry 76 :149-160
-
Dissolved carbon leaching from soil is a crucial component of the net ecosystem carbon residual. Global Change Biology 17 :1167-1185
-
More replenishment than priming loss of soil organic carbon with boosted carbon input. Nature Communications 9 :3175
-
Stabilization of microbial residues in soil organic matter later two years of decomposition. Soil Biological science and Biochemistry 141 :107687
graysonwhantem1946.blogspot.com
Source: https://peerj.com/articles/9128/
0 Response to "Review Ja P O N E S Q U E Foundation Brush"
ارسال یک نظر